
Enter the number of valence electrons, number of lone and bonded pairs. The calculator will readily determine the formal charge.
A proper price is described as:
“The person rate of each atom present in a molecule”
Figuring out the formal charge is of incredible importance. this is as it shows how reactive a molecule is and how it'll behave whilst developing bonds with both atoms.
This specific shape presentations the bonding electron pairs of atoms in a molecule. additionally, the presence of lone pairs inside the molecules is also proven the usage of this sample.
In Lewis shape:
Regardless of how complex the dot structure of a molecule is, the loose online formal fee calculator assists you in calculating price in a fragment of seconds.
| Element | Valence Electrons (V) | Lone Pair Electrons (LP) | Bonded Electrons (BE) | Formal Charge Formula | Formal Charge (FC) |
|---|---|---|---|---|---|
| Oxygen (O) in H₂O | 6 | 4 | 4 | FC = V - (LP + 0.5 × BE) | FC = 6 - (4 + 0.5 × 4) = 0 |
| Nitrogen (N) in NH₄⁺ | 5 | 0 | 8 | FC = 5 - (0 + 0.5 × 8) | FC = 5 - 4 = +1 |
| Carbon (C) in CO₂ | 4 | 0 | 8 | FC = 4 - (0 + 0.5 × 8) | FC = 4 - 4 = 0 |
Example 1
The way to determine formal fee on fluorine?
Solution:
The dot structure of fluorine is as beneath::
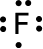
As we recognise that:
Valence shell electrons of sulphur = 7
Lone pair electrons of sulphur = 6
Bonded electrons of sulphur = 2
Calculating formal price:
$$ FC = V - \left(LP + 0.5BE\right) $$
$$ FC = 7 - \left(6 + 0.5*2\right) $$
$$ FC = 7 - \left(6 + 1\right) $$
$$ FC = 7 - \left(7\right) $$
$$ FC = 7 - 7 $$
$$ FC = 0 $$
Example 2
How to determine the formal charge on oxygen in a water (H₂O) molecule?
Solution:
Valence shell electrons of oxygen = 6
Lone pair electrons on oxygen = 4
Bonded electrons on oxygen = 4
Calculating formal charge:
$$ FC = V - \left(LP + 0.5BE\right) $$
$$ FC = 6 - \left(4 + 0.5 \times 4\right) $$
$$ FC = 6 - \left(4 + 2\right) $$
$$ FC = 6 - 6 $$
$$ FC = 0 $$
Thus, the formal charge on oxygen in a water molecule is 0.
Besides for the formulation, our loose on line formal rate calculator takes multiple seconds to generate correct results.
Make a use of this free calculator to calculate formal fee on any atom contained within the molecule. let’s find how!
Enter:
Output:
The unfastened formal rate calculator calculates:
According to the formal fee rule, the quantity of the electrons shared a few of the atoms of the molecule have to be in equilibrium. furthermore, this understanding also affords an edge in determining the proper Lewis structures..
whilst an atom donates a range of electrons and there's an octet shell vacant in them, then there exists a opportunity of gaining greater electrons to fill the octet shell. This gain of electrons consequences within the terrible formal charge on an atom.
No, the formal prices aren't the actual fees. they're just used to suppose the electrons transmission in among atoms for lewis systems.
Formal charge helps determine the most stable Lewis structure of a molecule. The form with the least official charges (nearest to nothing) normally appears the sturdiest. It also helps predict the reactivity of molecules in chemical reactions.
Count the valence electrons of the atom. Subtract the number of non-bonding electrons (lone pairs). Subtract half the number of bonding electrons (shared in covalent bonds). This aids in determining if an entity possesses a favorable, unfavorable, or balanced electrical state.
The formal charge and oxidation state are two systems we use in chemistry that look at electrons in different ways. Formal charge guesses that electrons shared between atoms in a bond are shared evenly. But oxidation state thinks that electrons are either given all away or swapped for another atom entirely. "Formal charge helps us draw Lewis diagrams. Oxidation number is used for redox reactions.
Drawing the Lewis structure properly means the formal charges should be as close to zero as you can and the negative charges on atoms that usually grab electrons. This results in a stable and realistic representation of the molecule.
Molecules with low formal charges are more stable. High formal charges indicate an unstable or highly reactive species. Reducing official accuses, scientists can ascertain the most plausible shape of a mixture.
Yes, an atom can have a positive or negative formal charge. A bad charge means the ball, which is an atom, got more tiny things called electrons, and a good charge means it lost them. These charges affect molecular polarity and reactivity.
Formal charge helps in choosing the best resonance structure of a molecule. The s that stick best have minimal charge and place negative charge on atoms that normally hold on tightly.
A refusal charge is a hypothetical notion used to facilitate the drawing of Lewis diagrams. It fails to depict the true charge spread within a molecule, a concept more accurately encapsulated by the nuanced portrayal of molecular orbitals' partial charges.